Decoding The Signal: How Trump's FDA Affects Biotech

Table of Contents
Accelerated Drug Approvals under Trump's FDA
The Trump administration prioritized accelerating drug approvals, implementing policies aimed at bringing life-saving medications to market more quickly. This approach, while lauded by some, also generated considerable controversy.
Right-to-Try Initiatives and their Impact
The expansion of "Right to Try" initiatives allowed terminally ill patients access to experimental treatments that hadn't completed the full FDA approval process. This had several significant implications:
- Increased patient access: Patients gained access to potentially life-extending therapies, offering hope in dire situations.
- Ethical considerations: The ethical implications of providing experimental drugs outside of controlled clinical trials sparked debate, particularly regarding informed consent and potential risks.
- Potential impact on clinical trial recruitment: Some argued that Right to Try might hinder clinical trial recruitment, as patients might opt for experimental treatments instead of participating in structured studies.
- Influence on drug pricing negotiations: The availability of experimental drugs through Right to Try could indirectly influence drug pricing negotiations and market dynamics.
Changes in Drug Approval Processes
The FDA implemented several changes to its regulatory pathways, notably accelerating the review processes for breakthrough therapies. These changes:
- Faster approvals for breakthrough therapies: This led to quicker market entry for potentially life-saving drugs, benefiting both patients and pharmaceutical companies.
- Potential risks associated with accelerated approvals: Critics raised concerns that the accelerated process might compromise safety and efficacy standards, leading to potential risks for patients.
- Impact on investor confidence: The faster approval times boosted investor confidence, leading to increased investment in promising biotech ventures.
Impact on Orphan Drug Development
Trump's FDA also focused on accelerating the approval of orphan drugs, which target rare diseases affecting small patient populations. This emphasis had several consequences:
- Increased incentives for orphan drug development: Companies were incentivized to invest in research and development for treatments addressing rare diseases, leading to a pipeline of new therapies.
- Financial implications for biotech firms: Developing orphan drugs can be financially challenging; the FDA's supportive policies mitigated some of these risks.
- Access to treatments for patients with rare diseases: Patients with rare diseases gained access to new treatments that were previously unavailable.
Regulatory Changes and their Influence on Biotech Investment
The changes implemented by Trump's FDA significantly impacted biotech investment and funding.
Impact on Biotech Funding and Investment
The altered regulatory landscape affected investment in biotech companies in several ways:
- Changes in venture capital investment: Faster approval pathways and increased investor confidence attracted significant venture capital funding to promising biotech startups.
- Impact on initial public offerings (IPOs): Successful drug approvals facilitated successful IPOs, providing biotech companies with crucial capital for growth.
- Effect on mergers and acquisitions (M&A) activity: Increased investment and the success of some biotech companies led to greater M&A activity within the sector.
Pricing and Reimbursement Policies
Changes in drug pricing policies under Trump's FDA also affected the financial viability of biotech companies.
- Negotiations with pharmaceutical companies: The administration engaged in negotiations with pharmaceutical companies to control drug prices, impacting profitability.
- Impact on profitability: While some companies benefited from faster approvals, the negotiations regarding drug pricing influenced their overall profitability.
- Potential effects on drug accessibility: Pricing policies played a role in determining the accessibility of newly approved drugs for patients.
Controversies and Criticism of Trump's FDA Approach
Despite the positive impacts, Trump's FDA approach faced significant criticism.
Concerns about Safety and Efficacy
A major concern was whether the accelerated approval process compromised safety and efficacy standards.
- Examples of controversial drug approvals: Specific drug approvals during this period sparked debate over the balance between speed and rigorous safety assessments.
- Public perception of safety: Concerns were raised about potential long-term consequences and the public's trust in the FDA's ability to ensure drug safety.
- Long-term consequences: The long-term effects of accelerated approvals on patient safety and public health remained a point of ongoing discussion.
Political Influence on Regulatory Decisions
Allegations of political influence on FDA decision-making further fueled controversy.
- Examples of potential political influence: Specific instances were cited where political pressure might have influenced regulatory decisions.
- Implications for transparency: Concerns were raised about the transparency and impartiality of the FDA's decision-making processes.
- Public trust in the regulatory system: The allegations eroded public trust in the integrity of the regulatory system.
Conclusion
Trump's FDA policies significantly impacted the biotech industry, leading to accelerated drug approvals, changes in investment patterns, and considerable controversy. The faster approval processes, while potentially benefiting patients by providing quicker access to innovative treatments, also raised concerns about compromised safety standards and the influence of politics on regulatory decisions. The impact on biotech investment was substantial, with increased venture capital and M&A activity, but the long-term consequences of these policies are still unfolding. Further understanding Trump's FDA's influence on biotech innovation requires careful analysis of individual drug approvals and the long-term effects on patient safety and the overall healthcare system. Continue decoding the signals to deepen your understanding of how Trump's FDA impacted biotech innovation and its lasting legacy.

Featured Posts
-
 Depenses Militaires Usa Russie Une Analyse De La Situation Par John Plassard Usa Today
Apr 23, 2025
Depenses Militaires Usa Russie Une Analyse De La Situation Par John Plassard Usa Today
Apr 23, 2025 -
 Land Your Dream Private Credit Role 5 Dos And Don Ts To Follow
Apr 23, 2025
Land Your Dream Private Credit Role 5 Dos And Don Ts To Follow
Apr 23, 2025 -
 The Hegseth Trump Leak Controversy A Deep Dive
Apr 23, 2025
The Hegseth Trump Leak Controversy A Deep Dive
Apr 23, 2025 -
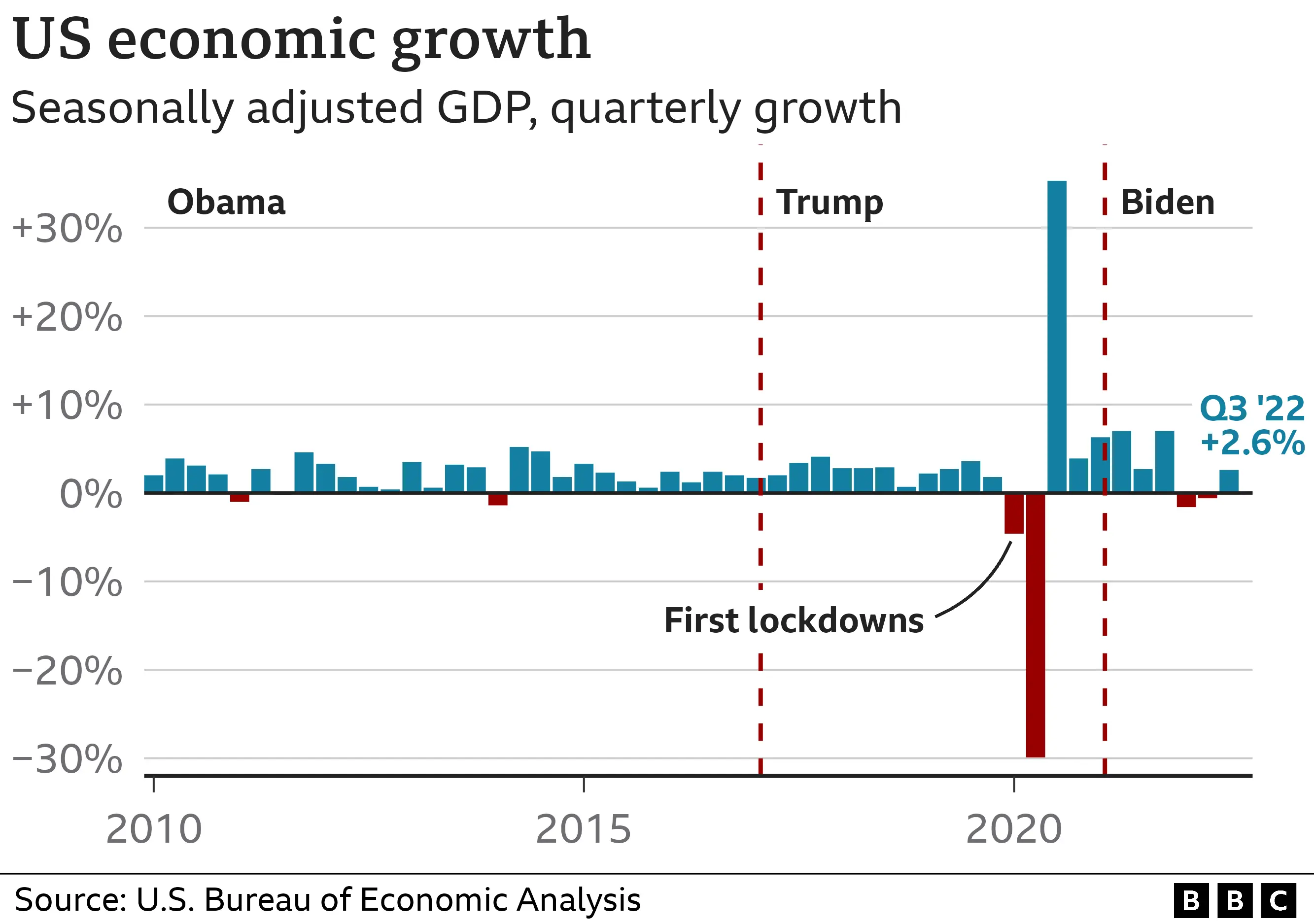 The Trump Presidency And Economic Performance A Data Driven Analysis
Apr 23, 2025
The Trump Presidency And Economic Performance A Data Driven Analysis
Apr 23, 2025 -
 Brewers Record Setting Nine Stolen Bases Lead To Dominant Win Against As
Apr 23, 2025
Brewers Record Setting Nine Stolen Bases Lead To Dominant Win Against As
Apr 23, 2025
