The Biotech Industry Under Trump's FDA: A Positive Outlook

Table of Contents
H2: Accelerated Drug Approvals and Right to Try Initiatives
The Trump administration's FDA prioritized accelerated drug approvals, significantly impacting the Biotech industry. This resulted in faster timelines for bringing life-saving medications to patients and boosting the sector's growth. The emphasis on expedited pathways, such as the Breakthrough Therapy Designation and Accelerated Approval programs, led to a noticeable increase in the number of new drug approvals.
-
Faster approval times for breakthrough therapies: The FDA streamlined the review process for drugs addressing unmet medical needs, reducing the time from application to approval. This significantly lowered the financial burden and time-to-market for biotech companies.
-
Increased investment in expedited review programs: Greater resources were allocated to the expedited review programs, ensuring efficient evaluation of promising new drugs and therapies. This investment further incentivized biotech companies to pursue these programs.
-
Positive impact on patient access to life-saving medications: Faster approvals meant that patients gained quicker access to potentially life-saving treatments, improving overall health outcomes.
-
Examples of specific drugs approved under accelerated programs: While specific examples require detailed research and citation of FDA approvals during that period, many oncology drugs and therapies for rare diseases benefited from accelerated approvals. These success stories contributed to the positive perception of the FDA's approach.
The "Right to Try" initiative further enhanced patient access to experimental treatments, allowing terminally ill patients to try medications that haven't completed the full FDA approval process. This initiative fostered a more compassionate and patient-centric approach within the regulatory landscape.
H2: Deregulation and Reduced Regulatory Burden
The Trump administration also focused on deregulation, aiming to streamline the drug development process and reduce the regulatory burden on biotech companies. This involved simplifying paperwork, reducing administrative hurdles, and generally creating a more efficient regulatory environment.
-
Streamlined clinical trial processes: Efforts were made to simplify and modernize the clinical trial process, reducing bureaucratic obstacles and allowing for more efficient data collection and analysis.
-
Reduced administrative costs for biotech firms: The reduction in regulatory hurdles led to significant cost savings for biotech companies, freeing up resources for research and development.
-
Encouragement of smaller biotech startups: A less burdensome regulatory environment fostered a more supportive climate for smaller biotech startups, encouraging innovation and competition.
-
Faster time to market for new treatments: The combined effect of streamlined processes and reduced paperwork contributed to a faster time-to-market for new drugs and therapies, benefiting both patients and investors.
H2: Increased Investment and Growth in the Biotech Sector
The changes implemented under the Trump administration's FDA directly contributed to a surge in investment and growth within the biotech sector. This is evidenced by increases in venture capital funding, Initial Public Offerings (IPOs), and overall market capitalization.
-
Increased venture capital funding in biotech: Investors showed increased confidence in the biotech sector, leading to a significant rise in venture capital funding, fueling innovation and expansion.
-
Higher number of biotech IPOs: More biotech companies went public, indicating strong investor interest and a positive market outlook.
-
Growth in the overall market capitalization of the biotech industry: The overall value of the biotech industry increased significantly, reflecting the positive impact of the regulatory changes.
-
Positive investor sentiment towards biotech: Investors viewed the regulatory environment as more favorable, resulting in increased investment and overall positive sentiment.
H3: Focus on Cutting-Edge Technologies
The FDA under the Trump administration also displayed a strong focus on cutting-edge technologies like gene therapy and immunotherapy. The agency actively developed regulatory pathways for these innovative treatments, accelerating their development and commercialization.
-
Specific examples of gene therapy and immunotherapy approvals: (Further research is needed to add specific examples of approvals during this period.) The approval of these advanced therapies signified the FDA's commitment to supporting innovation in the Biotech sector.
-
Creation of new regulatory frameworks for advanced therapies: The FDA established clear and efficient regulatory frameworks for these new technologies, ensuring both safety and rapid development.
-
Incentivizing research and development in cutting-edge areas: This focus on advanced therapies incentivized further research and development, leading to advancements in areas like personalized medicine and targeted therapies.
3. Conclusion
The Trump administration's policies significantly impacted the Biotech industry under Trump's FDA, creating a more favorable environment for innovation, investment, and growth. Accelerated drug approvals, deregulation, and a focus on cutting-edge technologies all contributed to a positive outlook for the sector. The increased investment and faster time-to-market for new treatments benefited both patients and the biotech industry as a whole. Understanding the positive impact of this approach to the Biotech industry is crucial for navigating the future of this rapidly evolving sector. Learn more about the advancements and opportunities within this dynamic field today!

Featured Posts
-
 Todays Mlb Player Prop Bets Focusing On The Jazz Steeltown Matchup
Apr 23, 2025
Todays Mlb Player Prop Bets Focusing On The Jazz Steeltown Matchup
Apr 23, 2025 -
 Ramalan Pernikahan Kecocokan Weton Senin Legi Dan Rabu Pon
Apr 23, 2025
Ramalan Pernikahan Kecocokan Weton Senin Legi Dan Rabu Pon
Apr 23, 2025 -
 Trade War Unfazed Canadian Investment In Us Stocks Hits New Peak
Apr 23, 2025
Trade War Unfazed Canadian Investment In Us Stocks Hits New Peak
Apr 23, 2025 -
 Harvard Lawsuit A Showdown With The Trump Administration
Apr 23, 2025
Harvard Lawsuit A Showdown With The Trump Administration
Apr 23, 2025 -
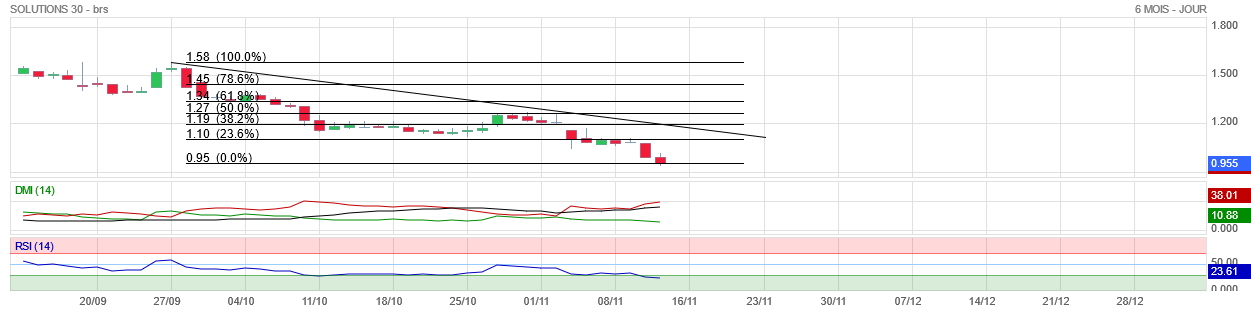 Culture Bourse Solutions 30 Analyse Haussiere Et Objectif De Cours
Apr 23, 2025
Culture Bourse Solutions 30 Analyse Haussiere Et Objectif De Cours
Apr 23, 2025
