Sanofi Chlamydia Vaccine Candidate Receives Fast Track Designation From US FDA
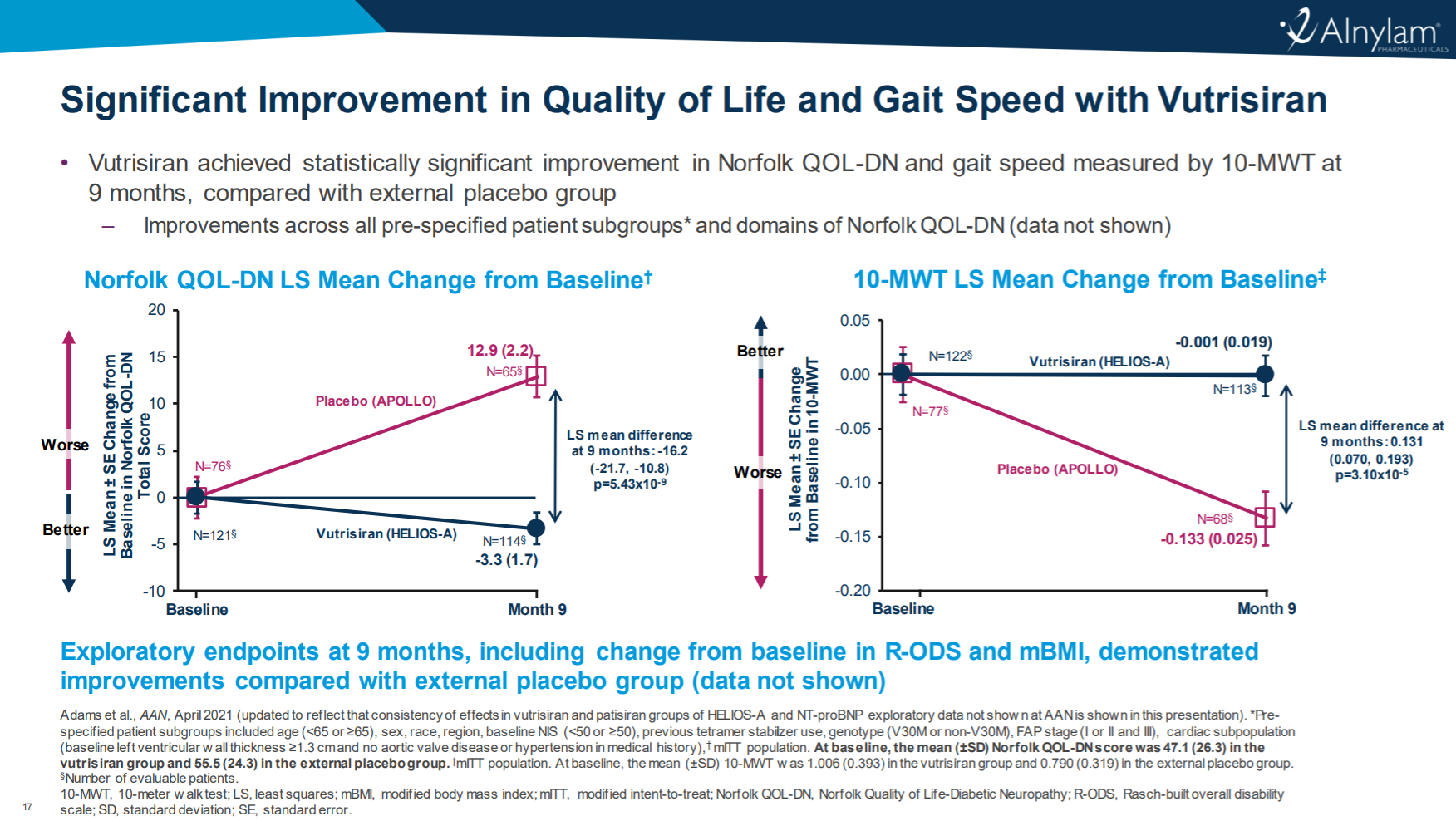
Table of Contents
Understanding the Significance of FDA's Fast Track Designation
The FDA's Fast Track Designation is a process designed to expedite the development and review of new drugs that treat serious conditions and fill an unmet medical need. For Sanofi's chlamydia vaccine candidate, this designation translates into several key advantages:
- Prioritized Review: Sanofi will receive more frequent meetings and interactions with the FDA throughout the development process. This ensures smoother communication and faster resolution of any arising issues.
- Accelerated Approval: If the vaccine candidate meets predefined criteria, it may be eligible for priority review and potentially accelerated approval, significantly reducing the time it takes to reach the market.
The benefits for Sanofi are substantial:
- Faster development timelines: The Fast Track program streamlines the regulatory pathway, leading to quicker progress toward clinical trials and eventual approval.
- Increased efficiency in clinical trials: The FDA's guidance and support help optimize the design and execution of clinical trials, minimizing delays and maximizing efficiency.
- Potential for earlier market access: This means patients could gain access to a potentially life-changing chlamydia vaccine much sooner than with standard regulatory pathways.
- Enhanced regulatory guidance: Sanofi benefits from more frequent and proactive communication with the FDA, receiving clear guidance on the data needed for approval.
The Urgent Need for a Chlamydia Vaccine
Chlamydia trachomatis, the bacteria causing chlamydia, is a significant global health concern. The infection's high prevalence and serious long-term consequences underscore the critical need for a preventative vaccine.
- High prevalence and serious health consequences: Chlamydia is one of the most commonly reported STIs worldwide, leading to significant health issues like infertility, ectopic pregnancies, and pelvic inflammatory disease (PID) if left untreated.
- Limitations of current treatment: Current treatment relies heavily on antibiotics, but antibiotic resistance is a growing concern, and reinfection rates remain high. This highlights the urgent need for a preventive measure like a vaccine.
The current situation is further complicated by:
- High rates of asymptomatic infection: Many individuals infected with chlamydia experience no symptoms, leading to delayed diagnosis and treatment, contributing to the spread of the infection.
- Antibiotic resistance concerns: The overuse of antibiotics has led to the emergence of antibiotic-resistant strains of chlamydia, making treatment more challenging.
- Long-term health risks: Untreated chlamydia poses significant long-term health risks for both men and women, impacting reproductive health and overall well-being.
- Significant social and economic burden: The high prevalence and associated healthcare costs associated with chlamydia place a substantial burden on healthcare systems and society.
Sanofi's Chlamydia Vaccine Candidate: Details and Potential
While specific details about Sanofi's vaccine candidate remain confidential, it is understood that they are pursuing a novel approach. The exact nature of the vaccine (e.g., DNA, protein-based) and details of its mechanism of action are likely to be published in peer-reviewed journals as clinical trials progress. The current stage of clinical trials and expected timelines will also be made public in the future.
The potential impact of a successful chlamydia vaccine is vast:
- Mechanism of action: The vaccine will likely work by stimulating the immune system to produce antibodies that can prevent or clear the infection.
- Results from previous clinical trial phases: As these become available, they will provide critical data on the vaccine's efficacy and safety.
- Target population: The vaccine will likely target sexually active individuals at high risk of chlamydia infection.
- Potential efficacy and safety profiles: These will be critical factors in determining the vaccine's suitability for widespread use.
Implications for Chlamydia Prevention and Treatment
A successful chlamydia vaccine would revolutionize STI prevention and management. The potential benefits extend far beyond individual health:
- Decreased incidence of chlamydia: The most significant impact would be a substantial reduction in the number of new chlamydia infections globally.
- Reduced healthcare costs: Lower infection rates would translate into decreased healthcare spending related to diagnosis, treatment, and management of chlamydia complications.
- Improved sexual health outcomes: Individuals would experience improved sexual health, reducing anxiety and concerns related to this common STI.
- Potential for integration into national vaccination programs: A safe and effective vaccine could be readily incorporated into existing national immunization schedules.
Conclusion
Sanofi's receipt of Fast Track Designation for its chlamydia vaccine candidate signifies a monumental step towards combating this widespread STI. This accelerated development pathway highlights the urgency and importance of developing a safe and effective chlamydia vaccine. The potential benefits – from decreased infection rates to reduced healthcare costs – are substantial and offer a beacon of hope for improving global sexual health.
Call to Action: Stay informed about the progress of Sanofi's chlamydia vaccine and other advancements in chlamydia prevention and treatment. Learn more about protecting yourself and your community from this prevalent infection. Follow the latest news on the Sanofi chlamydia vaccine development.

Featured Posts
-
 Thompsons Unlucky Monte Carlo Run A Detailed Look
May 31, 2025
Thompsons Unlucky Monte Carlo Run A Detailed Look
May 31, 2025 -
 Psg Vs Inter Milan A Champions League Final Preview
May 31, 2025
Psg Vs Inter Milan A Champions League Final Preview
May 31, 2025 -
 Houstons Unexpected Crisis Rats And Drug Addiction
May 31, 2025
Houstons Unexpected Crisis Rats And Drug Addiction
May 31, 2025 -
 Severe Storms Possible Across Carolinas Tracking Active Vs Expired Weather Alerts
May 31, 2025
Severe Storms Possible Across Carolinas Tracking Active Vs Expired Weather Alerts
May 31, 2025 -
 Understanding The Drug Addiction Crisis Among Houstons Rats
May 31, 2025
Understanding The Drug Addiction Crisis Among Houstons Rats
May 31, 2025
