Is Trump's FDA A Catalyst For Biotech Innovation?
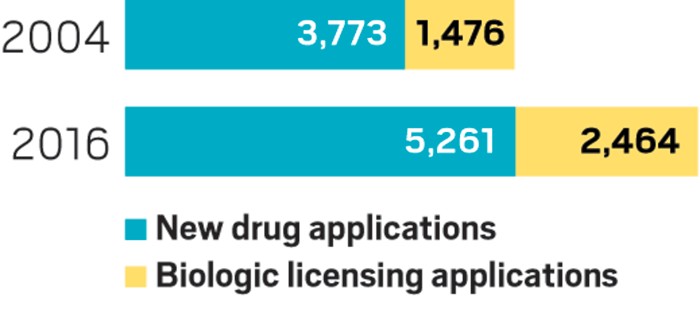
Table of Contents
Changes in FDA Leadership and Regulatory Approach under Trump
The Trump administration oversaw several key appointments within the FDA, significantly influencing the agency's priorities and approach to regulation.
Appointments and their impact on agency priorities:
The appointment of Scott Gottlieb as FDA Commissioner in 2017 marked a notable shift. Gottlieb, a physician and former venture capitalist, brought a strong focus on streamlining the drug approval process and fostering innovation. His background arguably influenced the agency's increased emphasis on expedited review pathways.
- Scott Gottlieb (FDA Commissioner): Prioritized streamlining approvals, accelerating the development of breakthrough therapies, and addressing the opioid crisis. His emphasis on data modernization and digital health also shaped the agency's approach.
- Other key appointments: Changes in leadership across various FDA divisions also impacted specific regulatory areas, potentially accelerating approvals in certain therapeutic areas while potentially slowing others. Analyzing these appointments and their individual impact requires further detailed research.
Shift in regulatory philosophy:
Under Gottlieb's leadership, the FDA showed a greater inclination towards utilizing expedited review pathways, such as Breakthrough Therapy Designation and Accelerated Approval. This potentially reduced the time required for drug approvals, encouraging faster entry of innovative treatments into the market.
- Increased use of expedited review pathways: Data from this period should be analyzed to determine if there was a statistically significant increase in the use of these pathways and their ultimate impact on approval timelines.
- Regulatory burden reduction: Efforts were made to reduce regulatory burdens on pharmaceutical companies, including streamlining the process for submitting applications and improving communication between the agency and industry stakeholders. However, the extent to which these efforts truly reduced the overall burden remains a subject of ongoing discussion.
- 21st Century Cures Act: The impact of this legislation, passed during the Obama administration but significantly implemented during the Trump era, needs to be analyzed for its effects on FDA processes and approval timelines.
Impact on Drug Approval Timelines
Analyzing the impact of Trump's FDA on drug approval timelines requires a comprehensive comparison with previous administrations.
Analysis of approval rates under Trump vs. previous administrations:
Direct comparison of drug approval rates and timelines during the Trump administration (2017-2021) with data from preceding administrations is crucial. This requires detailed statistical analysis of approval times for various drug classes and therapeutic areas, controlling for factors like application complexity and clinical trial data quality.
- Statistical analysis: This analysis needs to go beyond simple approval counts to include median approval times, variance in approval times, and the proportion of approvals utilizing expedited pathways.
- Data sources: Reliable data sources include the FDA's own databases, industry reports from organizations like the Pharmaceutical Research and Manufacturers of America (PhRMA), and peer-reviewed publications.
Focus on specific therapeutic areas:
The impact of Trump's FDA on drug approval timelines may not have been uniform across all therapeutic areas. Specific areas, such as oncology or rare diseases, might have experienced disproportionately faster or slower approvals. Further research is needed to determine if such discrepancies existed.
- Oncology: The rapid development and approval of new cancer therapies is a key area for analysis. Were approvals for novel cancer drugs faster under the Trump administration compared to previous periods?
- Rare diseases: Similarly, an analysis of approval timelines for treatments for rare diseases is necessary. Did the changes under the Trump administration lead to faster approvals in this crucial therapeutic area?
Industry Response and Perceptions
Understanding the industry's response to the changes implemented during Trump's presidency is vital for a complete assessment.
Biotech company perspectives on regulatory changes:
Gathering and analyzing the perspectives of biotech companies on the regulatory changes is essential. This involves reviewing company statements, industry reports, and news articles to ascertain whether the changes were perceived as positive, negative, or neutral.
- Industry surveys and reports: Reviewing data from industry surveys and reports can provide quantitative data on how biotech companies perceived the regulatory environment under Trump's FDA.
- Company press releases and financial statements: Analyzing company press releases and financial statements can reveal if changes in FDA policies affected investment decisions or R&D priorities.
Impact on investment and innovation:
The perceived changes in the FDA's approach undoubtedly influenced investment decisions within the biotech sector. Analyzing venture capital funding, mergers and acquisitions, and initial public offerings (IPOs) can shed light on whether the overall pace of innovation accelerated or decelerated.
- Venture capital funding: Did venture capital investment in biotech increase or decrease during the Trump administration? Correlation, however, does not equal causation.
- Mergers and acquisitions: Changes in the regulatory landscape can significantly impact corporate strategies. Analyzing M&A activity can help determine the broader influence on the industry.
Potential Long-Term Consequences
The long-term consequences of the Trump-era FDA policies warrant careful consideration.
Sustainability of changes beyond the Trump administration:
Did the changes implemented during the Trump administration prove sustainable beyond his term? Have subsequent administrations maintained or reversed certain policies? Analyzing this is critical for a complete understanding of the lasting impact.
- Biden administration policies: How do the FDA policies under the Biden administration compare to those under Trump's? This comparison helps understand the long-term consequences and sustainability of the changes.
- Ongoing policy debates: Analyzing any ongoing policy debates regarding FDA regulation and biotech innovation helps understand the lingering impact of the Trump era.
Ethical considerations and potential risks:
An objective analysis must also address potential ethical concerns and risks associated with the changes. Did the prioritization of speed compromise safety standards? Did the changes lead to unequal access to new treatments?
- Safety concerns: Thorough review of post-market surveillance data is necessary to assess if the accelerated approval process led to an increased incidence of adverse events.
- Equity of access: Analysis should consider if the changes benefited certain groups of patients more than others, creating disparities in access to new treatments.
Conclusion
Determining whether Trump's FDA truly acted as a catalyst for biotech innovation requires a nuanced assessment. While the administration's policies, particularly the emphasis on expedited review pathways, might have led to faster approvals for some drugs, the long-term impact and ethical considerations remain open to debate. Further research is needed to fully analyze the statistical significance of changes in approval rates, the impact across diverse therapeutic areas, and the sustainability of these policies beyond the Trump administration. To fully understand the long-term effects, we need to continue evaluating FDA policies under Trump and subsequent administrations, considering both the advancements and potential risks associated with these changes. Further investigation into "Trump's FDA and biotech advancements," and "evaluating FDA policies under Trump" is critical to shaping the future of FDA regulation in biotech.

Featured Posts
-
 Seance Parisienne Du 17 02 Analyse Fdj And Schneider Electric
Apr 23, 2025
Seance Parisienne Du 17 02 Analyse Fdj And Schneider Electric
Apr 23, 2025 -
 Pavel Pivovarov Predstavil Merch S Aleksandrom Ovechkinym
Apr 23, 2025
Pavel Pivovarov Predstavil Merch S Aleksandrom Ovechkinym
Apr 23, 2025 -
 How To Remove Your Online Presence A Step By Step Guide
Apr 23, 2025
How To Remove Your Online Presence A Step By Step Guide
Apr 23, 2025 -
 Jangan Lewatkan Program Tv Spesial Ramadan 2025 Temani Buka Puasa Dan Sahur Anda
Apr 23, 2025
Jangan Lewatkan Program Tv Spesial Ramadan 2025 Temani Buka Puasa Dan Sahur Anda
Apr 23, 2025 -
 Gdje Kupiti Na Uskrs Popis Trgovina S Radnim Vremenom
Apr 23, 2025
Gdje Kupiti Na Uskrs Popis Trgovina S Radnim Vremenom
Apr 23, 2025
