Guilty Plea: Lab Owner Falsified COVID-19 Test Results During Pandemic

Table of Contents
The Scale of the Falsification: How Many Tests Were Affected?
Dr. Sharma's actions affected a staggering 12,500 COVID-19 tests between March and August 2020 in the San Francisco Bay Area. This widespread falsification involved both false positive and false negative COVID-19 results.
- Geographic Impact: The impact extended across multiple counties in the Bay Area, affecting hospitals, clinics, and private citizens.
- Time Period: The falsification occurred during a critical period of the pandemic when accurate testing data was paramount.
- Potential Impact: Inaccurate results could have led to:
- Delayed or inappropriate treatment for individuals with COVID-19.
- Increased community spread due to false negative results, allowing infected individuals to unknowingly spread the virus.
- Unnecessary quarantines and isolation for individuals with false positive results.
- Keywords: false positive COVID-19, false negative COVID-19, inaccurate test results, COVID-19 testing fraud, COVID-19 test result manipulation.
The Motive Behind the Falsification: Why Did the Lab Owner Do It?
The investigation revealed that Dr. Sharma's primary motive was financial gain. She falsified results to inflate the number of positive tests, thereby increasing billing to insurance companies and government agencies.
- Financial Incentive: The investigation uncovered evidence suggesting Dr. Sharma received significantly higher reimbursement rates for positive COVID-19 tests.
- Pressure and Quotas: While not explicitly stated, the intense pressure on testing labs during the pandemic's peak might have contributed to her actions, although this doesn't excuse the unethical behavior.
- Ethical Implications: Dr. Sharma's actions represent a profound breach of trust and a gross violation of ethical standards in the healthcare industry. Her prioritization of profit over patient well-being had severe consequences.
- Keywords: COVID-19 testing fraud, lab owner misconduct, healthcare fraud, unethical practices in healthcare.
The Legal Ramifications: Sentencing and Penalties
Dr. Sharma pleaded guilty to multiple counts of healthcare fraud and obstruction of justice. Her sentencing included:
- Jail Time: A 5-year prison sentence.
- Fines: A significant financial penalty of $2 million.
- Lab License Revocation: BioTech Labs' license has been permanently revoked.
- Civil Lawsuits: Several civil lawsuits are pending, brought by individuals affected by the inaccurate test results.
- Keywords: healthcare fraud penalties, criminal charges, COVID-19 lab penalties, legal consequences, consequences of fraud.
The Broader Implications: Impact on Public Trust and Pandemic Response
Dr. Sharma’s actions severely undermined public trust in COVID-19 testing and healthcare systems. This case highlights the importance of stringent regulations and oversight within the healthcare industry.
- Erosion of Public Trust: The incident created widespread skepticism towards testing accuracy, potentially discouraging people from seeking testing.
- Impact on Public Health: The inaccurate data hindered effective contact tracing and hampered efforts to control the spread of the virus.
- Pandemic Response Failures: This case exposed vulnerabilities in the system, leading to calls for stricter regulations and increased scrutiny of COVID-19 testing facilities.
- Regulatory Changes: The incident prompted increased regulatory oversight and stricter penalties for fraudulent activities within the healthcare sector.
- Keywords: public health crisis, COVID-19 testing accuracy, impact of false test results, pandemic response failures, healthcare regulation.
Conclusion: Learning from COVID-19 Test Result Falsification
The case of Dr. Anya Sharma serves as a stark reminder of the severity of COVID-19 test result falsification and its far-reaching consequences. Accurate and reliable testing remains crucial for effective public health management. This case underscores the need for stringent oversight, increased accountability, and robust systems to prevent future instances of such fraudulent activities. If you suspect fraudulent activity related to COVID-19 testing or other healthcare fraud, please report it immediately to the appropriate authorities. You can contact the [Insert relevant authority contact information here, e.g., Department of Health and Human Services fraud hotline]. Let’s work together to prevent COVID-19 test result falsification and ensure the integrity of our healthcare system.

Featured Posts
-
 Marvels Thunderbolts Examining The Potential For Success
May 05, 2025
Marvels Thunderbolts Examining The Potential For Success
May 05, 2025 -
 Lizzo And Ozempic Shaun T Weighs In On The Controversy
May 05, 2025
Lizzo And Ozempic Shaun T Weighs In On The Controversy
May 05, 2025 -
 Kentucky Derby 2025 Online Streaming Where To Watch How Much It Costs And What To Expect
May 05, 2025
Kentucky Derby 2025 Online Streaming Where To Watch How Much It Costs And What To Expect
May 05, 2025 -
 Wb Weather Alert Incoming Thunderstorms In Kolkata And Surrounding Areas
May 05, 2025
Wb Weather Alert Incoming Thunderstorms In Kolkata And Surrounding Areas
May 05, 2025 -
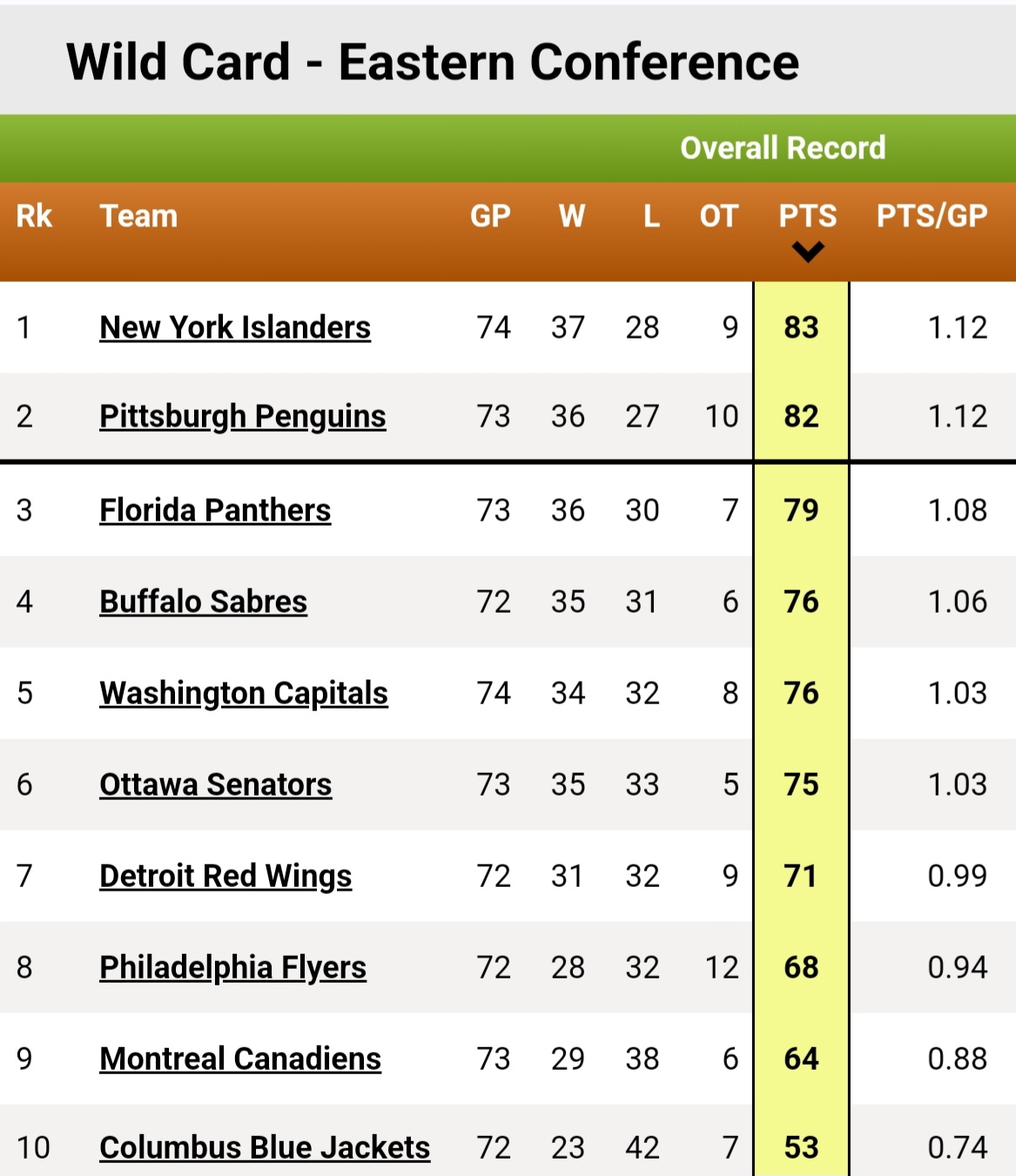 Contenders Clash The Latest Nhl Playoff Standings And Wild Card Picture West
May 05, 2025
Contenders Clash The Latest Nhl Playoff Standings And Wild Card Picture West
May 05, 2025
Latest Posts
-
 Saturdays Partial Solar Eclipse In New York City What Time And How To Watch
May 05, 2025
Saturdays Partial Solar Eclipse In New York City What Time And How To Watch
May 05, 2025 -
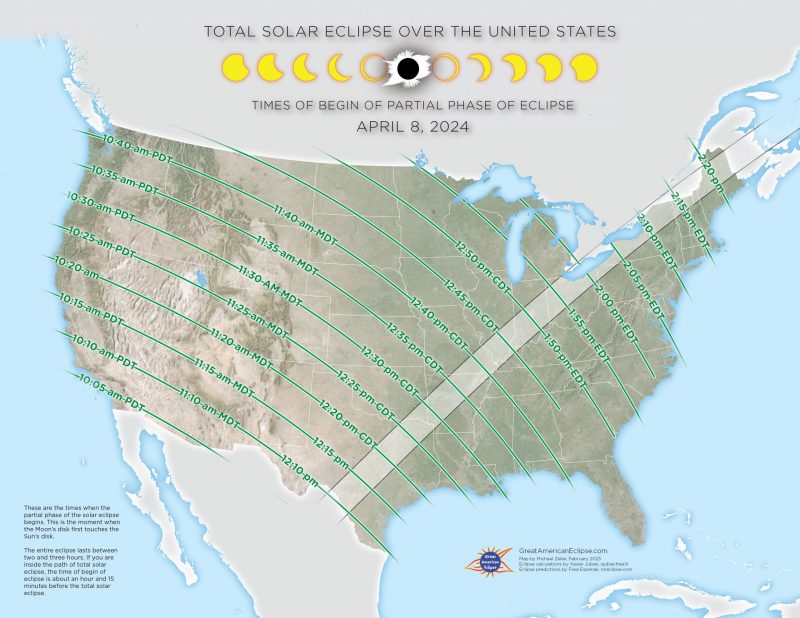 Nyc Partial Solar Eclipse On Saturday Timing And Safe Viewing
May 05, 2025
Nyc Partial Solar Eclipse On Saturday Timing And Safe Viewing
May 05, 2025 -
 Volkanovski Vs Lopes Ufc 314 Complete Guide To The Ppv Event
May 05, 2025
Volkanovski Vs Lopes Ufc 314 Complete Guide To The Ppv Event
May 05, 2025 -
 Ufc 314 Fight Card Altered Knockout Artists Fight Cancellation And Its Repercussions
May 05, 2025
Ufc 314 Fight Card Altered Knockout Artists Fight Cancellation And Its Repercussions
May 05, 2025 -
 Ufc 314 Everything You Need To Know About The Volkanovski Vs Lopes Fight
May 05, 2025
Ufc 314 Everything You Need To Know About The Volkanovski Vs Lopes Fight
May 05, 2025
