Advanced Gene Editing For Precise Complete Gene Integration
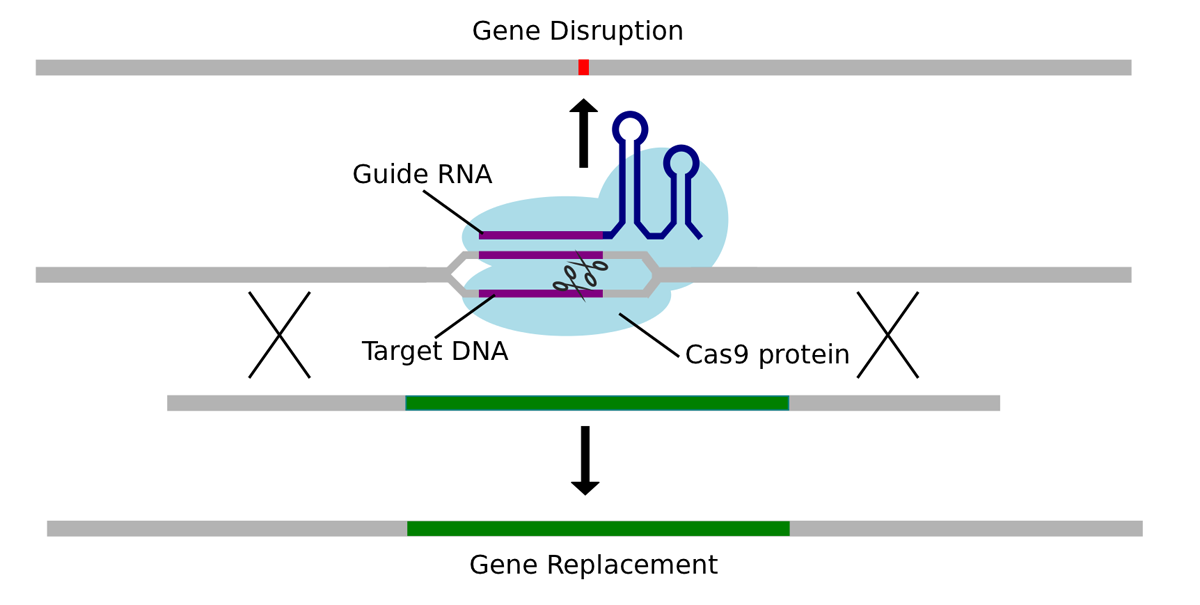
Table of Contents
Precise Gene Targeting Techniques
Precise gene targeting is the cornerstone of successful gene therapy. Achieving complete and accurate integration of a therapeutic gene requires sophisticated tools and strategies.
CRISPR-Cas Systems & Enhancements
CRISPR-Cas systems, particularly CRISPR-Cas9, have revolutionized gene editing. However, achieving precise complete gene integration requires careful consideration of various factors. Base editing and prime editing represent significant advancements over traditional CRISPR-Cas9, offering higher precision and reduced off-target effects.
- CRISPR-Cas9: While highly efficient, it can cause unintended double-strand breaks and off-target edits.
- Base Editing: Allows for precise single-base changes without double-strand breaks, minimizing off-target effects.
- Prime Editing: A more versatile technique enabling a wider range of edits, including insertions and deletions, with potentially higher precision.
Mitigation strategies for off-target effects include improved guide RNA design, the use of high-fidelity Cas enzymes, and the implementation of screening methods to detect and minimize unintended edits. Successful applications include correcting genetic defects in cell cultures and animal models.
Homologous Recombination (HR) Optimization
Homologous recombination (HR) is a natural cellular process that facilitates precise gene integration. However, the efficiency of HR is often low. Several strategies aim to enhance HR-mediated gene integration:
- Donor DNA Design: Optimizing the design of the donor DNA template, including homology arms length and sequence, significantly improves integration efficiency.
- Recombinase Enzymes: Employing recombinase enzymes, such as Cre-loxP or FLP-FRT systems, can increase the rate of homologous recombination.
- Cellular Factors: Manipulating cellular factors that influence HR, such as DNA repair proteins, can also boost integration efficiency.
Despite these advancements, achieving high HR rates remains a significant challenge, particularly in non-dividing cells.
Non-viral Delivery Systems for Precise Gene Integration
Efficient delivery of gene editing tools and donor DNA into target cells is crucial for successful gene therapy. Non-viral delivery methods offer advantages over viral vectors in terms of safety and scalability.
- Lipid Nanoparticles: These versatile carriers can encapsulate both Cas enzymes and donor DNA, delivering them effectively to various cell types.
- Electroporation: This technique uses electrical pulses to create temporary pores in cell membranes, allowing entry of gene editing components.
- Microinjection: Direct injection of gene editing components into single cells is highly precise but less scalable.
The efficacy of each method varies depending on the cell type and tissue being targeted, and each presents its own limitations in terms of delivery efficiency and potential toxicity.
Monitoring and Validating Complete Gene Integration
Accurate assessment of gene integration is crucial for evaluating the success of gene editing therapies.
Advanced Detection Methods
Several advanced methods are used to verify successful and complete gene integration:
- PCR: A rapid and relatively inexpensive method for detecting the presence of integrated DNA.
- Southern Blotting: A more sensitive technique used to confirm the size and number of integrated copies.
- Next-Generation Sequencing (NGS): Enables comprehensive analysis of the genome, allowing identification of the integration site and any off-target effects.
- Fluorescence In Situ Hybridization (FISH): A cytogenetic technique used to visualize the location of the integrated gene within the cell.
The choice of method depends on the sensitivity and specificity required for the application.
Quantitative Assessment of Integration Efficiency
Quantifying the percentage of successfully modified cells is crucial for determining the efficacy of gene editing therapies.
- Flow Cytometry: Used to analyze the percentage of cells expressing a marker gene linked to the integrated therapeutic gene.
- Digital PCR: A highly sensitive method for quantifying the number of copies of the integrated gene.
Accurate quantification is essential for determining the therapeutic potential of gene editing approaches.
Addressing Challenges in Complete Gene Integration
Despite advancements, several challenges remain in achieving precise and complete gene integration:
Off-target Effects and Minimization Strategies
Off-target edits, where the gene editing machinery modifies unintended genomic locations, are a major concern.
- Improved Guide RNA Design: Using algorithms to design guide RNAs with high specificity reduces off-target effects.
- Improved Cas Enzyme Engineering: Engineered Cas enzymes with increased fidelity and reduced off-target activity are being developed.
- Screening Methods: Comprehensive screening methods are employed to identify and quantify off-target edits.
Minimizing off-target effects is critical for the safety and efficacy of gene editing therapies.
Immunogenicity of Gene Editing Tools
The immune system can recognize and mount an immune response against gene editing components, potentially leading to adverse effects.
- Immune Modulation: Strategies to suppress or modulate the immune response can mitigate immunogenicity.
- Engineering Less Immunogenic Components: Modifying the structure of gene editing components to reduce their immunogenicity is an active area of research.
- Patient Selection Criteria: Careful selection of patients based on their immune profile can minimize the risk of immune responses.
Overcoming immunogenicity is critical for the long-term success of gene editing therapies.
Long-Term Stability of Integrated Genes
The integrated gene needs to remain stable and expressed over time for long-term therapeutic benefit.
- Regulatory Elements: Using strong and stable regulatory elements can ensure long-term expression of the integrated gene.
- Epigenetic Modifications: Addressing epigenetic modifications that might lead to gene silencing is crucial for maintaining long-term expression.
Conclusion: The Future of Precise Gene Integration and its Therapeutic Potential
Advanced Gene Editing for Precise Complete Gene Integration has made remarkable progress, with new techniques and strategies constantly emerging. Precise and complete gene integration is paramount for the success of gene therapy, offering the potential to cure a wide range of genetic diseases. While challenges remain, including minimizing off-target effects, addressing immunogenicity, and ensuring long-term stability, ongoing research and development in this field are paving the way for safer and more effective gene therapies. We encourage further research and development in advanced gene editing techniques to improve gene therapy outcomes and address unmet medical needs. To explore further resources and related articles, please visit our website.

Featured Posts
-
 Air Jordan May 2025 Sneaker Release Roundup
May 30, 2025
Air Jordan May 2025 Sneaker Release Roundup
May 30, 2025 -
 Cancelacion Axe Ceremonia 2025 Como Reclamar Tu Reembolso En Ticketmaster
May 30, 2025
Cancelacion Axe Ceremonia 2025 Como Reclamar Tu Reembolso En Ticketmaster
May 30, 2025 -
 Cancelacion Axe Ceremonia 2025 Reclama Tu Reembolso Ticketmaster
May 30, 2025
Cancelacion Axe Ceremonia 2025 Reclama Tu Reembolso Ticketmaster
May 30, 2025 -
 Will Dana White Pay Jon Jones 29 Million A Ufc Veterans Prediction
May 30, 2025
Will Dana White Pay Jon Jones 29 Million A Ufc Veterans Prediction
May 30, 2025 -
 Bruno Fernandes Futuro Em Questao Apos Contato Do Al Hilal
May 30, 2025
Bruno Fernandes Futuro Em Questao Apos Contato Do Al Hilal
May 30, 2025
